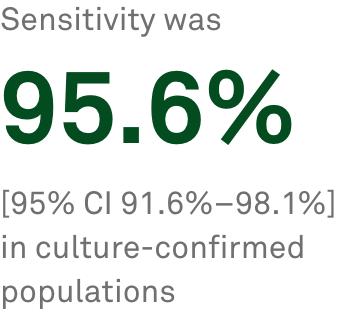
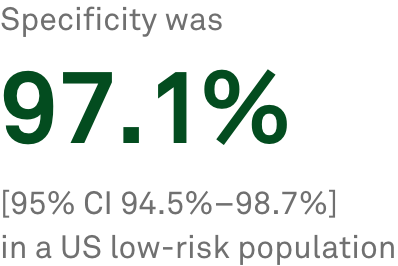
T-SPOT.TB delivers objective results for a broader patient population
The T-SPOT.TBa blood test provided by Quest Diagnostics offers a higher standard in latent TB testing than subjective skin testing, delivering objective results for a broader population.
TB blood testing is ideal for:
- General population including patients 2+ years old
- Patients with compromised immune systems1
- Bacillus Calmette-Guérin (BCG)–vaccinated patients2
a Quest Diagnostics has validated the use of this assay under CLIA for processing specimens more than 8 hours after collection, up to 54 hours.
The T-SPOT®.TB test is an in vitro diagnostic test for the detection of effector T cells that respond to stimulation by Mycobacterium tuberculosis antigens ESAT-6 and CFP-10 by capturing interferon gamma (IFN-γ) in the vicinity of T cells in human whole blood collected in sodium citrate or sodium or lithium heparin. It is intended for use as an aid in the diagnosis of M tuberculosis infection. The T-SPOT.TB test is an indirect test for M tuberculosis infection (including disease) and is intended for use in conjunction with risk assessment, radiography, and other medical and diagnostic evaluations.
Up-to-date relevant warnings, precautions, side effects, and contraindications can be found at: http://www.oxfordimmunotec.com/north-america/
The CPT® codes provided are based on American Medical Association guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payer being billed.
Test codes may vary by location. Please contact your local laboratory for more information.
References:
- Komiya K, Ariga H, Nagai H, et al. Impact of peripheral lymphocyte count on the sensitivity of 2 IFN-gamma release assays, QFT-G and ELISPOT, in patients with pulmonary tuberculosis. Intern Med. 2010;49(17):1849-1855. doi:10.2169/internalmedicine.49.3659
- CDC. Fact sheets: tuberculin skin testing. Updated November 2, 2020. Accessed March 9, 2022. https://www.cdc.gov/tb/publications/ factsheets/testing/skintesting.htm